Engine oils. What does my oil actually do?
An engine oil’s job is primarily to stop all the metal surfaces in your engine from grinding together and tearing themselves apart from friction whilst transferring heat away from the combustion cycle. Engine oil must also be able to hold all the nasty by-products of combustion, such as silica (silicon oxide) and acids in suspension. It cleans the engine of these chemicals and build-ups, and keeps the moving parts coated in oil. Finally, engine oil minimises the exposure to oxygen and thus oxidation at higher temperatures. It does all of these things under tremendous heat and pressure.
How much do you value the engine in your car?
Think about it, because the life of your engine depends in no small part on the quality of the oil you put in it – oil is the lifeblood of your car’s engine. From the mid 80’s for 8 or 9 years there was a veritable revolution in car engine oil. All oils were no longer the same thanks to the popularity of hot hatches, 16-valve engines and turbos as the tuner scene started to rise. Combined with the devastating problems of black death, the days of one oil catering for everyone were over.
Take Castrol for example. They led the field for years with GTX. This was surpassed a few years back by semi-synthetic and fully synthetic oils, including GTX2 and GTX3 Lightec. Now, that’s been surpassed by Formula SLX which can cost upwards of £50 for 5 litres. And most recently, Castrol GTX Magnatec which is muscling in on the hitherto separate world of friction reducers
What was Black Death?
Black Death first appeared in the early 80’s when a horrible sticky black substance was found to be the cause of many engine seizures in Europe. Faster roads, higher under-hood temperatures, tighter engineering tolerances and overworked engine oils turned out to be contributors to the problem. The oils just couldn’t handle it and changed their chemical makeup under pressure into a sort of tar-like glue. This blocked all the oil channels in the engines, starved them of lubrication and caused them to seize. I don’t recommend this but you can reproduce the effect with a frying pan, cooking oil and a blowtorch. The cooking oil will heat up far quicker than it’s designed to and will turn to a sticky black tar in your pan. Either that or it will set fire to your kitchen, which is why I said “don’t do this”.
Anyway, burning kitchens aside, Black Death was the catalyst for the production of newer higher quality oils, many of them man-made rather than mineral-based.
Black death for the 21st century
There’s a snappy new moniker for Black Death now, and it’s called sludge. The cause is the same as Black Death and it seems to be regardless of maintenance or mileage. The chemical compounds in engine oils break down over time due to prolonged exposure to high temperatures and poor maintenance habits. When the oil oxidises, the additives separate from the oil and begin to chemically break down and solidify, leading to the baked-on oil deposits turning gelatinous, and that nasty compound is what is lovingly referred to nowadays as sludge. It’s like black yoghurt. What doesn’t help is that modern engines, due to packaging, have smaller sumps than in the “good old days” and so hold less oil. This means that the oil that is present in the engine can’t hold as much crap (for want of a better word) and can lead to earlier chemical breakdown.
The most common factor in sludge buildup is mineral oils combined with a lack of maintenance by the car owner combined with harsh driving conditions. But this isn’t true in all cases. For some reason, a 2005 Consumer Reports article discovered that some engines from Audi, Chrysler, Saab, Toyota, and Volkswagen appear prone to sludge almost no matter how often the oil is changed.
Â
Mineral or synthetic?
Mineral oils are based on oil that comes from dear old Mother Earth which has been refined. Synthetic oils are entirely concocted by chemists wearing white lab coats in oil company laboratories. For more info, see the section on synthetics further down the page. The only other type is semi-synthetic, sometimes called premium, which is a blend of the two. It is safe to mix the different types, but it’s wiser to switch completely to a new type rather than mixing.
Synthetics
Despite their name, most synthetic derived motor oils (ie Mobil 1, Castrol Formula RS etc ) are actually derived from mineral oils – they are mostly Polyalphaolifins and these come from the purest part of the mineral oil refraction process, the gas. PAO oils will mix with normal mineral oils which means Joe public can add synthetic to his mineral, or mineral to his synthetic without his car engine seizing up (although I’ve heard Mobil 1 is actually made by reformulating ethanol).
The most stable bases are polyol-ester. When I say ‘stable’ I mean ‘less likely to react adversely with other compounds.’ Synthetic oil bases tend not to contain reactive carbon atoms for this reason. Reactive carbon has a tendency to combine with oxygen creating an acid. As you can imagine, in an oil, this would be A Bad Thing. So think of synthetic oils as custom-built oils. They’re designed to do the job efficiently but without any of the excess baggage that can accompany mineral based oils.
 A quick guide to the different grades of oil.
| Fully Synthetic | Characteristics |
|---|---|
| 0W-30 0W-40 5W-40 |
Fuel economy savings Enhances engine performance and power Ensures engine is protected from wear and deposit build-up Ensures good cold starting and quick circulation in freezing temperatures Gets to moving parts of the engine quickly |
| Semi-synthetic | Characteristics |
| 5W-30 10W-40 15W-40 |
Better protection Good protection within the first 10 minutes after starting out Roughly three times better at reducing engine wear Increased oil change intervals – don’t need to change it quite so often |
| Mineral | Characteristics |
| 10W-40 15W-40 |
Basic protection for a variety of engines Oil needs to be changed more often |
Viscosity and Viscosity Index (VI).
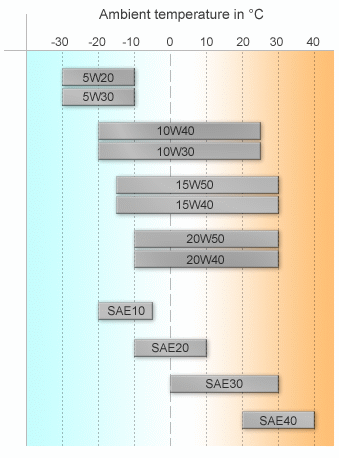
The proper viscosity is the single most important criteria of a lubricating oil. The basic performance of machinery is based on the viscosity of the lubricant. Viscosity is, if you like, the resistance to the flowability of the oil. The thicker an oil, the higher its viscosity. The chart on the right shows a rough guide to ambient temperatures vs oil viscosity performance in both multigrade (top half) and single grade (lower half) oils.
Multigrade oils work by having a polymer added to a light base oil which prevents the oil from thinning too much as it warms up. At low temperatures, the polymers are coiled up and allow the oil to flow as it’s low number (W number) indicates. As the oil heats up, the polymers unwind into long chains which prevent the oil from thinning as much as it normally would. The result is that at 100°C, the oil has thinned only as much as it’s higher rating. Think of it like this: a 10W30 oil is a 10-weight oil that will not thin more than a 30-weight oil when it gets hot.
The viscosity index of a lubricant is an empirical formula that allows the change in viscosity in the presence of heat to be calculated. This tells the user how much the oil will thin when it is subjected to heat. The higher the viscosity index, the less an oil will thin at a specified temperature. Multi-viscosity motor oils will have a viscosity index well over 100, while single viscosity motor oils and most industrial oils will have a VI of about 100 or less.